The toxicity of PAEs remains under debate because the debate is characterized by tension between the commercial importance of PAEs and their impact on human health and on the environment. - Net et al. (2015)
1 What are phthalates?
Phthalates are a suite of chemicals that share the base structure of a benzene ring and alkyl chains of varying lengths. They are commonly added to consumer products. Lower molecular weight phthalates are primarily used as solvents in cosmetics while higher molecular weight phthalates are added to plastics like polyvinyl chloride (PVC) to increase flexibility.
“Plasticizers work by reducing the chemical affinity between molecules when embedded between chains of plastic raw materials (or act as monomers in polycarbonate plastic), but as plasticizers are not especially stable in these products, they can leach out and thus end up in the environment. Phthalates are not classified as persistent compounds (Staples et al.1997), but their occurrence in the environment has been reported widely, possibly arguing against a rapid biodegradation in some environments (Fatoki & Vernon 1990; Bauer & Herrmann 1997; Heemken et al. 2001; Fromme et al. 2002; Beauchesne et al.2007)” - Oehlmann et al. (2009)
25 phthalate congeners are currently manufactured
6 Phthalates identified by the U.S. EPA as priority pollutants:
DMP (dimethyl phthalate) & DEP (diethyl phthalate)1, BBP (benzyl-butyl phthalate), DBP (dibutyl phthalate), DEHP (diethyl hexyl phthalate), DnOP (di-n-ocytl phthalate)
1 water soluble solvents used more in cosmetics than in plastic products
Since we are starting from the drawing board I’m going to do a quick review of the literature and look for environmental phthalate concentrations found in near-coastal waters, specifically I’m interested in water soluble phthalates like di-methyl phthalate (DMP) and di-ethyl phthlate (DEP). Francesco Saliu suggested we focus on water soluble phthalates in our experimental design so that we don’t have to mess with a carrier solvent control, but can still control the treatments to be within a range of environmental relevance. However, I just learned that “Low molecular weight PAEs such as dimethyl phthalate (DMP), diethyl phthalate (DEP), and DnBP are components of industrial solvents, solvents in perfumes, adhesives, waxes, inks, pharmaceutical products, insecticide materials, and cosmetics (Net et al. 2015)” and that PAEs with longer alkyl chains are used as plasticizers. Since I want to tie my work into marine plastic pollution, I should really focus on common phthalates added to plastics. Unfortunately, it looks like using a water soluble PAE may not be an option.
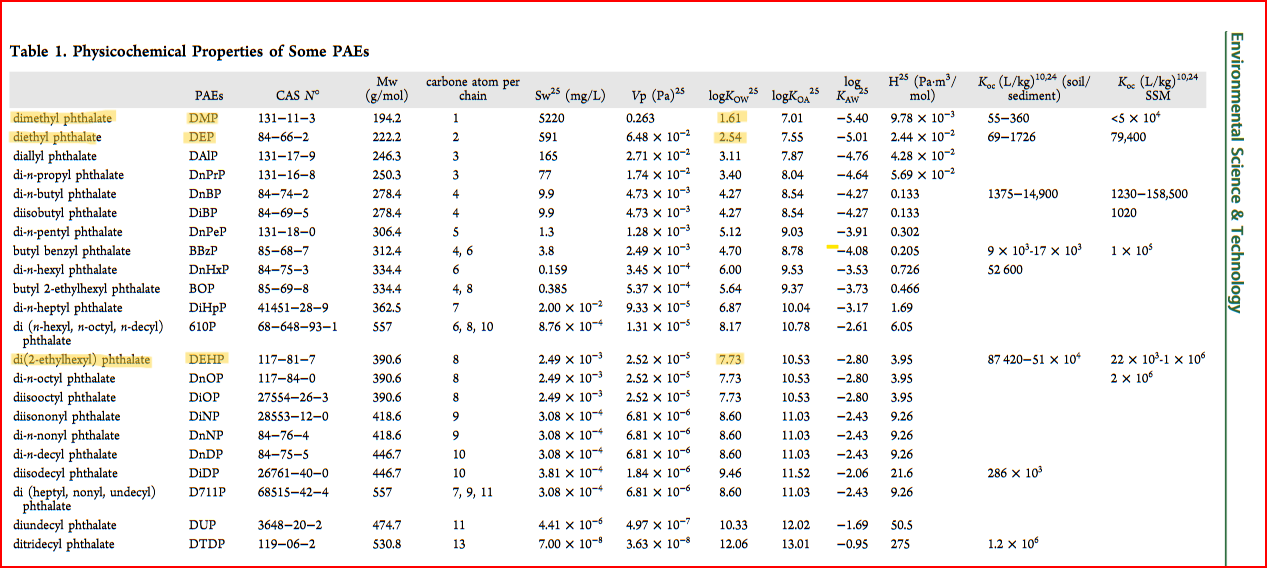
In the table from Net et al. (2015), water solubility (Sw25 ) indicates solubility in mg/L at 25\(^\circ\)C. Notice that it decreases with increasing alkyl length (carbon atom per chain, or carbon chain length).
I learned about the concept of octanol-water partition coefficient (Kow), which is a log value used for “predicting how a chemical will concentrate in marine organisms.” A high value of Kow indicates higher potential bioconcentration in organisms, explained in further detail in Net et al. (2015) and Amézqueta et al. (2020).
The half-life of PAEs decrease with temperature!!!!
Longer half-lives are more likely under anaerobic conditions and in cold, nutrient poor environments.
The half-life of PAEs decrease in the presence of photosensitizers like nitrate, nitrite and chromophoric dissolved organic matter (CDOM)2
2 CDOM is the fraction of dissolved organic carbon pool that absorbs light in both the ultra violet and visible ranges. It is sometimes called ‘coloured’ dissolved organic matter. The decay of organic matter releases tannins that stain the water, CDOM are the main light-absorbing particles in aquatic systems and estimated to account for 20-70% of dissolved organic carbon in the ocean, with particularly high values of CDOM in coastal zones with heavy terrestrial input (Zhao et al. 2023).
Even if PAEs can be eliminated from different environmental matrices via various processes as reported in previous sections, their extensive use and permanent emissions have resulted in their ubiquitous presence in the environment. Net et al. (2015)
2 Environmental pollution of phthalates in marine waters
I found a good table by Hermabessiere et al. (2017) that shows a review of papers that report levels of phthalates in marine waters.. however most of the sampling sites are in Europe and the Mediterranean. I’d like to expand on the table to get a better global picture of phthalate pollution levels in coastal marine waters.
Law, Fileman, and Matthiessen (1991) conducted sampling around the North and Irish Seas in 1988 and 1989. In this short and sweet report, values are represented as ng/dm3 which is nanograms per cubic decimeter.
“The marine sediments contained about half the concentration as the freshwater lake sediments, but in both matrices DINP had the highest concentrations of all analytes. DINP has been found at higher concentrations than other phthalates in sediments of urban areas, which has been suggested to be due to its higher environmental persistence and rising use following DEHP restrictions (Björklund et al., 2009).” (Mathieu and Bednarek, 2022, p. 23) (Mathieu and Bednarek 2022)
3 Ecological Relevance
predicted no-effect concentrations (PNEC)
“Due to the lack of ecotoxicity thresholds in the United States, results from this study were compared to lowest predicted no-effects concentrations (PNECs) estimated from the European Union’s NORMAN Ecotoxicology database.5 The EU PNECs are based on either experimental ecotoxicity data or quantitative structure-activity relationship (QSAR) predictions where empirical data is lacking. A measured environmental concentration that exceeds a lowest PNEC is considered by European Union member states to warrant further review for regulatory concern. These values are not considered robust thresholds; rather they are agreed-upon values by NORMAN experts to be used for preliminary prioritization of chemicals. When available, additional thresholds are used in this report, such as the sediment management standards (SMS) values for sediment cleanup objectives (SCO) and cleanup screening levels (CSLs) (Ecology, 2013; WAC 173-204).” (Mathieu and Bednarek 2022)
4 Quantifying phthalates
Survey of Phthalates in Washington State Waterbodies, 2021 (Mathieu and Bednarek 2022)
- analytes:
- matrix: freshwater
- Sample Prep: EPA 3541
- Sample Clean-up: EPA 3620C florisil
- Analytical method: EPA 8270E (GC/MS)
- Lab: Manchester Environmental Laboratory
Plastic-derived contaminants in Aleutian Archipelago seabirds with varied foraging strategies (Padula et al. 2020)
- analytes: DMP (dimethyl phthalate), DEP (diethyl phthalate), BBP (benzyl-butyl phthalate), DBP (dibutyl phthalate), DEHP (diethyl hexyl phthalate), DnOP (di-n-ocytl phthalate)
- matrix: seabird tissue
- Sample Prep:
- “We followed best practices and removed plastic materials from the area of the lab where phthalate analyses were performed and checked potential sources for system contamination such as solvents, instrument blanks, air, and laboratory glassware. We washed glassware with hexane and acetone and ashed it at 440 °C for 2 h before use (Fankhauser-Noti and Grob, 2007). For each individual bird, 3–6 g of muscle sample were homogenized and stored in a glass vial. A Quencher method with primary secondary amine (PSA), Supelclean (Sigma-Aldrich 52738-U), magnesium sulfate, and sodium chloride was used for extracting phthalates from animal tissue. Samples were weighed into 10-mL Kimax glass centrifuge tubes together with a mixture of MgSO4 and NaCl (Martins et al., 2016). One hundred nanograms of the surrogates DMP-d4 and DEHP-d4 and 2 mL of acetonitrile were added to the samples. DMP-d4 and DEHP-d4 are deuterated analogs of two of the targeted compounds in this study, and were utilized as internal standards to maintain precise quantification of phthalates (Net et al., 2015). Samples were then vortexed for 1 min and placed in an ultrasonic bath for 20 min and kept at 4 °C for 24 h to complete the extraction. Next, samples were vortexed for 5 min and centrifuged. The liquid was siphoned off and placed in a second glass culture tube that had been previously filled with MgSO4 and PSA sorbent resin to remove other organic compounds. The mixture was vortexed for 3 min and centrifuged. Next, 0.5 mL of the supernatant liquid was filtered and transferred to a 2-mL autosampler vial with Teflon septa, and internal standards DBP-d4 and DnOP-d4 were added at a concentration of 50 ng/mL.”
- Analytical Method:
- HPLC (Agilent 1200) connected to a tandem mass spectrometer (Agilent 6410B) equipped with an ESI source.
- Phase separation was achieved with a Zorbax SB-C18 2.1 to 30 mm, 3.5 mm analytical column and XDB-C18 4.6 to 12.5, 5 mm guard column.
- Mobile phases were water (A) and methanol (B) with 10 mM formic acid (B = 25–85% in 5 min, 85–90% in 9 min; B = 90% for 9 min; mass spectrometer settings: gas temperature = 300 °C; gas flow = 6 L/ min; nebulizer = 15 psi; capillary voltage = 4000 V).
- Quality control samples, method blanks, extraction blanks, solvent blanks, instrument blanks, and continuous calibration verification standards were analyzed every 10th sample and sample duplicates were analyzed with every extraction batch (approximately 20 samples).
- In addition, spiked samples and blanks were carried through the entire extraction procedure to determine method recovery.
- Phthalate concentration was determined using external seven-level calibration using ratios of internal standards and recovery of surrogate was recorded to assure extraction recovery.
- Limit of detection (LOD) was calculated from seven-level calibrations following Harris (2010)
- Lab: Applied Science, Engineering & Technology (ASET) laboratory, University of Alaska Anchorage
WA Dept. of Ecology Analytical Lab Search
ACZ Laboratories, Inc., Steamboat Springs, CO
GEL Laboratories, LLC., Charleston, SC
King County Environmental Laboratory, Seattle, WA
Tacoma Environmental Services Laboratory, Tacoma, WA
Manchester Environmental Laboratories